Rapid Viral Detection Systems LLC
Access
Information
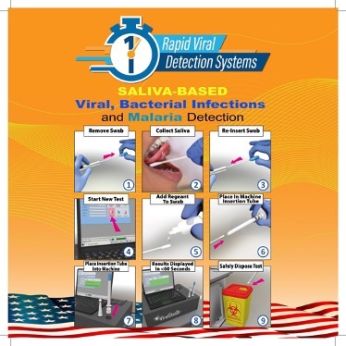
Rapid Viral Diagnostic Systems (RVDS) is a biotechnology InteGen-affiliated company with a focus on and a track record of developing innovative tools. It has developed proprietary, clinically validated technologies (Ion Mobility Spectrometry) which allow for the rapid detection (23 seconds) and diagnosis of several infectious and tropical diseases to meet the unmet need for instantaneous point-of-care (POC) testing and diagnosis. The patent-pending tests will revolutionize POC diagnostics by offering a cost effective, noninvasive (saliva based), and highly accurate test without the need for physician administration. Currently RVDS’ rapid test, ViralStatDx TM can detect malaria, TB, hepatitis, HIV/AIDS, COVID-19, flu and RSV 60 times faster than traditional methods. Research is ongoing to expand the test portfolio to include additional infectious and non-infectious diseases.
InteGen is a diagnostic reagent manufacturing company that has developed disruptive technologies, including innovative DNA probes with clinical applications in cancer genetics, prenatal diagnoses, miscarriages, and IVF. InteGen’s InstaFish technology provides results of up to 10 targets in a single assay in less than1 hour, using our extremely cost-effective multi-color and multiplex probe design. Our newest product, OncoSureTM can detect all human cancers in less than 2 hours using a non-invasive 3cc peripheral blood sample. It uses powerful screening technology that can detect cancers much earlier and faster than expensive and invasive serum marker and imaging tests (e.g. PSA, CT, and PET scan). OncoSureTM can also monitor cancer treatment efficacy in 100% concordance with clinical remission. The test has the following characteristics: Sensitivity (96.88%), Specificity. (92.31%), Positive Predictive Value (81.58%), Negative Predictive Value (98.82%) and Accuracy/Area Under the Curve (AUC 0.944).
All DNA probes are manufactured in our Orlando facility which is FDA compliant with quality system 21CFR820 and cGMP. Our FDA registration number is #3011310310
To learn more, visit our Booth: USA Partnership Pavilion E-310G